noble gas configuration for copper|Electronegativity and chemical hardness of elements under : Tuguegarao Mar 1, 2022 — Furthermore, we discover that pressure-induced s - d orbital transfer makes Ni, Pd, and Pt “pseudo–noble-gas” atoms with a closed d-shell configuration, and the . Discover the top online slots for real money at the best casino sites for 2024. Explore trusted platforms offering exciting slot games, secure transactions, and generous bonuses. Start spinning and winning today!
PH0 · using noble gas notation write the electron configuration for the
PH1 · physical chemistry
PH2 · Write the noble gas electron configuration for copper Cu
PH3 · Write the condensed (noble
PH4 · What is the noble gas configuration of a copper (I) ion? A)
PH5 · What is the noble gas configuration for copper?
PH6 · What is the electron configuration of copper?
PH7 · Noble gas configuration for copper
PH8 · Give the ground
PH9 · Electronegativity and chemical hardness of elements under
PH10 · Electron Configuration Chart of All Elements (Full Chart)
sewing techniques no Instagram: “#جشنواره #لباس #تکنیکهای_خیاطی #خلاقیت #تكنولوژى .
noble gas configuration for copper*******Dis 12, 2016 — Copper has an electron configuration of [Ar] 3dX10 4sX1 [ A r] 3 d X 10 4 s X 1. Now sometimes the noble state is written as [Ar] 3dX10 4sX1 [ A r] 3 d X 10 4 s X 1 or as [Ar] 4sX2 3dX9 [ A r] 4 s X 2 3 d X 9.
Ene 25, 2014 — Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for .Mar 1, 2022 — Furthermore, we discover that pressure-induced s - d orbital transfer makes Ni, Pd, and Pt “pseudo–noble-gas” atoms with a closed d-shell configuration, and the .
Mar 23, 2023 — The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Free Gift for you: Interactive Periodic Table. Let me tell you .
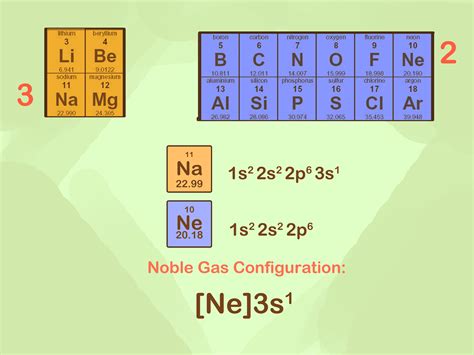
May 26, 2024 — The noble gas configuration for copper is [Ar] 3d10 4s1. Copper's electron configuration can be shortened by replacing the preceding energy levels with the noble .Copper (Cu) is a d-block transition metal with an atomic number of 29. The complete electronic configuration of Cu can be written as follows- 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹º 4s¹. .VIDEO ANSWER: There are two electron configurations that can be used to write for ions. The complete configuration for the copper 2 ion was written by the first 1 and we are .
Hul 14, 2024 —
The electron configuration for a neutral copper atom is [Ar] 4s2 3d9. The noble gas "Ar" stands for argon, which has an atomic number of 18. The use of noble .Nob 12, 2023 — The noble gas configuration of a copper (I) ion is [Ar]3d¹⁰. This configuration denotes a stable state for the Copper (I) ion, as it resembles a noble gas electron .noble gas configuration for copperAgo 19, 2023 — The ground-state electron configuration for copper (Cu) is [Ar]4s13d10. This is because copper is an exception to the Aufbau principle – it attains more stability .noble gas configuration for copper Electronegativity and chemical hardness of elements under Ago 19, 2023 — The ground-state electron configuration for copper (Cu) is [Ar]4s13d10. This is because copper is an exception to the Aufbau principle – it attains more stability .
Electronegativity and chemical hardness of elements under A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of \(\left[ \ce{Ne} \right]\) for the \(1s^2 2s^2 2p^6\) part of the configuration. Sodium's noble gas configuration becomes \(\left .Hun 30, 2024 — The noble gas configuration of copper (Cu) is [Ar] 3d^10 4s^1. The noble gas that would be listed is argon (Ar), which has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6.
Hul 27, 2021 — The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The .Write the condensed (noble-gas) electron configuration of copper. [He] [Ne] [Ar] [kr] Kr [Xe] [Rn] 1 2 3 4 5 6 क S pdf 0 0 0 1 00
Using NOBLE GAS notation write the electron configuration for the copper(II) ion. Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.

Give the noble gas electron configuration of the Ag+ ion. Of the ions below, only has a noble gas electron configuration. a) S3- b) O2+ c) I+ d) K- e) Cl-Write the electron configuration for a boron atom. Write the ground-state electron configuration and noble gas configuration for gallium. Write the condensed electron configuration for chromium.May 2, 2021 — What is the noble gas electron configuration of copper? Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for copper, 1s22s22p63s23p64s23d9 or in noble gas configuration [Ar] 4s23d9. However, because the 3d orbital is so much .Ago 19, 2023 — The ground-state electron configuration for copper (Cu) using noble-gas shorthand is [Ar]3d^104s^1. This configuration is due to the stability provided by a filled 3d subshell, which is an exception to the expected electron orbital filling order. The ground-state electron configuration for copper (Cu) using noble-gas shorthand is [Ar]3d104s1.
Nob 12, 2023 — The noble gas configuration of a copper(I) ion is [Ar]3d¹⁰. The noble gas configuration is a shorthand way of representing the electron arrangement within atoms. When copper transforms into a Cu+ ion, it loses an electron from its 4s orbital making it [Ar]3d¹⁰ much like the behavior in Zinc. This results in what is referred to as a pseudo .
Using NOBLE GAS notation write the electron configuration for the iron(III) ion. Show transcribed image text. There are 3 steps to solve this one. Step 1. Step-1. The electronic configuration can be written by using three basic rule . Using NOBLE GAS notation write the electron configuration for the iron(III) ion. Not the question you’re .
Hun 4, 2020 — Does Cu have a noble gas configuration? Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for copper, 1s22s22p63s23p64s23d9 or in noble gas configuration [Ar] 4s23d9 .
Using NOBLE GAS notation write the electron configuration for the copper(II) ion. Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
Write the condensed noble gas electron configuration for copper. Write the condensed noble gas electron configuration for Nb atom . Write the ground state electron configuration of Br using the noble-gas shorthand notation. Write the electron configuration of the following ions by using spdf notation: (a) Cr2+, Cr3+, Cr6+ ; (b) .Okt 31, 2023 — The condensed (noble-gas) electron configuration of copper can be written as [Ar] 3d¹⁰ 4s¹. To understand this electron configuration, let's break it down step by step: 1. Start with the noble gas before copper in the periodic table, which is argon (Ar). The electron configuration of argon is 1s² 2s² 2p⁶ 3s² 3p⁶.
Copper. Copper is a transition metal whose atomic number is 29. It's in the fourth row on the periodic table and is a very conductive metal. For this reason it is commonly used for electrical wiring.Using NOBLE GAS notation write the electron configuration for the copper atom. Write the complete electron configuration for the oxygen atom. . Using NOBLE GAS notation write the electron configuration for the calcium atom. Here’s the best way to solve it. View the full answer. Previous question Next question. Not the question you’re .Question: Write the complete electron configuration for the cobalt(III) ion. Using NOBLE GAS notation write the electron configuration for the chromium(III) ion. Write the complete electron configuration for the nickel(II) ion. Using NOBLE GAS notation write the electron configuration for the copper(I) ion.Noble-Gas Configuration: The ground-state electronic configuration of an atom of an element is the distribution of its electrons into different shells, subshells, and orbitals. The configuration and filling of electrons follow Aufbau's principle, Hund's rule, and Pauli's exclusion principle.
Svg Vector Icons : http://www.sfont.cn . Confirmation. Error
noble gas configuration for copper|Electronegativity and chemical hardness of elements under